Chemical Engineering Journal
First Author: Guo Ke
Corresponding Authors: Feng Hao, Li Qiang
Article Title: “A Full-Spectrum-Responsive Metal-Organic Framework-Derived Carbon-Supported Bimetallic Catalyst for Photothermal Catalytic Methane Dry Reforming”
Impact Factor: 13.2
Article Link: https://doi.org/10.1016/j.cej.2025.165155
Research background
The energy shortage and carbon emission crisis caused by fossil fuel depletion urgently need to be addressed. Solar-driven conversion of CO₂ and CH₄ into synthesis gas (CO/H₂) offers a strategy that combines environmental and energy benefits. While methane dry reforming (MDR) can convert greenhouse gases, it faces challenges such as thermodynamic barriers (ΔH = +247 kJ·mol⁻¹) and catalyst deactivation (severe carbon deposition on nickel-based catalysts). Traditional photocatalysis utilizes only a portion of the light spectrum, resulting in low efficiency. Photothermal catalysis, on the other hand, absorbs light across the entire spectrum, driving reactions through photothermal conversion. However, the development of catalysts that combine efficient light absorption, resistance to carbon deposition, and stability is crucial. This study, using MOFs as precursors, aims to construct highly efficient photothermal catalysts that achieve the dual goals of solar energy storage and greenhouse gas resource utilization.
Paper highlights/abstract
Photothermal methane reforming to carbon dioxide is an attractive process that enables the simultaneous conversion of solar energy and greenhouse gases. However, achieving high light-to-fuel efficiency remains a significant challenge due to the limited full-spectrum light absorption and catalytic activity of photothermal catalysts. Here, we report a metal-organic framework (MOF)-derived carbon-supported nickel-molybdenum bimetallic catalyst for photothermal methane reforming to carbon dioxide. The proposed photothermal catalyst exhibits excellent full-spectrum responsiveness. By controlling the spectral range and light intensity, we found that the reaction is primarily a light-driven thermochemical reaction and therefore relies significantly on efficient photothermal conversion. Furthermore, in situ Fourier transform infrared spectroscopy reveals that the introduction of relevant molybdenum species onto the nickel-based catalyst promotes the decomposition of carbon dioxide, thereby balancing the activation kinetics of methane and carbon dioxide and suppressing carbon deposition. The results showed that under direct illumination, the catalyst achieved excellent catalytic performance, with a carbon dioxide conversion rate of 61.2%, an advanced CO production rate of 141.7 mmol·g⁻¹·min⁻¹, a hydrogen-to-carbon ratio of 0.88, and a light-to-fuel efficiency of 36.8%, which is one of the highest values reported.
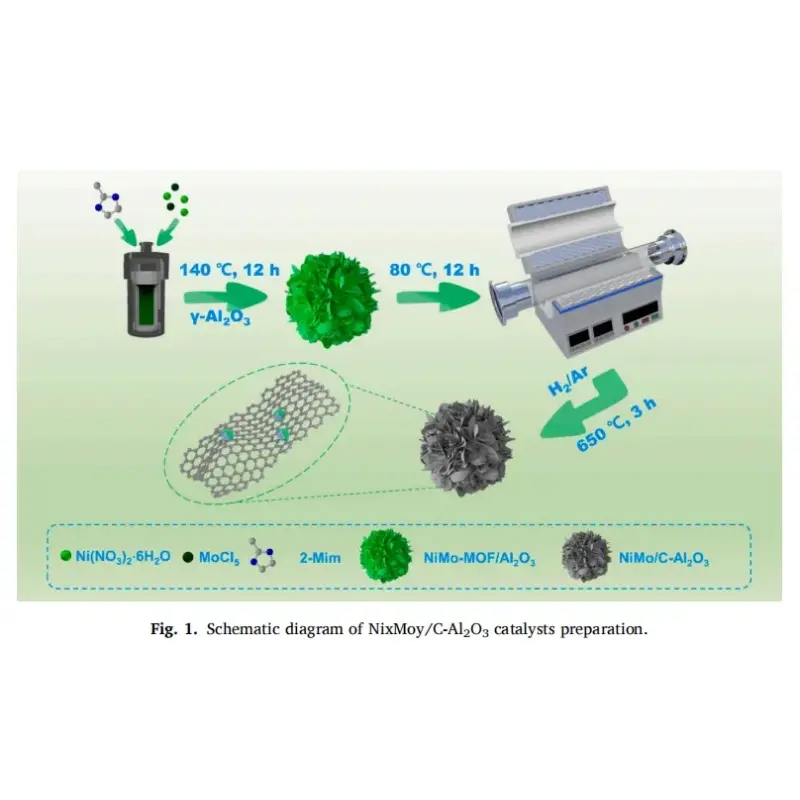
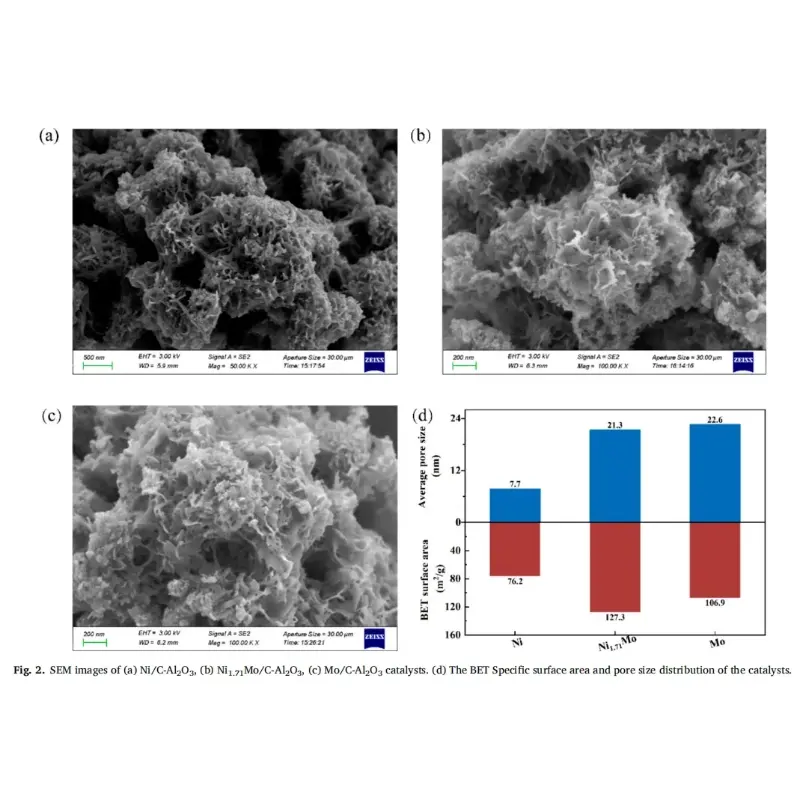
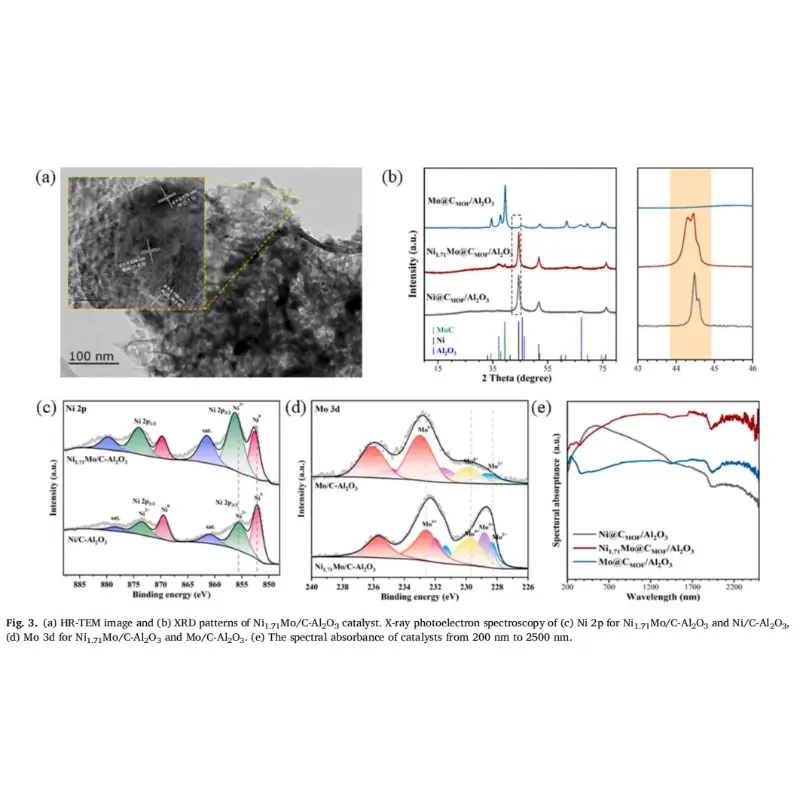
The customized high-temperature photocatalytic vertical furnace used by Professor Feng Hao’s research group in the experiment was provided by KeMi Instruments. Anhui KeMi Instruments Co., Ltd. was also specifically mentioned in the paper. We would like to express our sincere gratitude to Professor Feng Hao for his choice and recognition of KeMi Instruments.